CO2Carbon dioxide phase changes may occur when it is depressurised, depending on the initial and final pressure and temperature conditions. The depressurisation of CO2Carbon dioxide by design or by accident can result in the sublimation temperature of solid CO2Carbon dioxide. In addition, significant quantities of solid CO2Carbon dioxide can be formed within systems and/or within any release which in addition to its low temperature could cause blockages, and subsequent hazard. Having an adequate understanding of the thermodynamics of the CO2 streamA flow of substances resulting from CO2 capture processes, or which consists of a sufficient fraction of CO2 and sufficiently low concentrations of other substances to meet specifications of streams permitted for long term geological storage, including the effects of the impurities, is essential within the design and operation of CO2 streamA flow of substances resulting from CO2 capture processes, or which consists of a sufficient fraction of CO2 and sufficiently low concentrations of other substances to meet specifications of streams permitted for long term geological storage handling systems. Low temperatures could lead to the embrittlement of materials causing fractures and cracks (DNV, 2013).
CO2Carbon dioxide density is also sensitive to temperature changes especially close to critical pointThe highest temperature and pressure at which a substance can exist as a vapour and liquid phase in equilibrium conditions (i.e., 31 °C and 74 bars, see Fig. 2-3). This can lead directly to system over pressurisation with a relatively small change in CO2Carbon dioxide temperature. Appropriate system pressure relief should avoid this leading to a hazardous event (DNV, 2013).
| 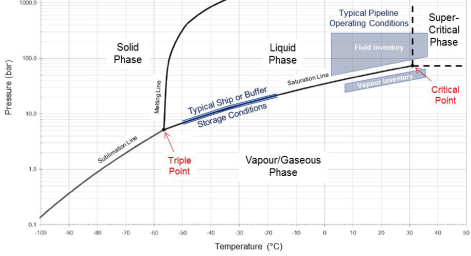
Fig. 2-3: CO2Carbon dioxide phase diagram with typical transportation conditions (DNV, 2013). |
A rupture of e.g a vessel containing large amounts of liquid CO2Carbon dioxide could lead to rapid pressure reductionThe gain of one or more electrons by an atom, molecule, or ion which under certain conditions could escalate to create a Boiling Liquid Expanding Vapour Explosion (BLEVE). The probability of this occurring is believed to be extremely low but CO2Carbon dioxide system designers should be aware of the potential (DNV, 2013).
Induced seismic activity has mainly been recognized along previously faulted rocks at waste disposal sites, oil fields, and other sites. Supercritical(CO2) Conditions where carbon dioxide has some characteristics of a gas and some of a liquid CO2Carbon dioxide liquid is less dense than water and may cause density-driven stress conditions at depth or interact with formation waterWater that occurs naturally within the pores of rock formations and rocks, causing a reductionThe gain of one or more electrons by an atom, molecule, or ion in permeabilityAbility to flow or transmit fluids through a porous solid such as rock and pressure build-up leading to seismic activity. Seismic events are unlikely to occur due to injectionThe process of using pressure to force fluids down wells in porous rocks unless very high injectionThe process of using pressure to force fluids down wells pressures cause hydraulic fracturing. Thorough characterisation, testing, and monitoringMeasurement and surveillance activities necessary for ensuring safe and reliable operation of a CGS project (storage integrity), and for estimating emission reductions of stress conditions at depth will prevent the riskConcept that denotes the product of the probability of a hazard and the subsequent consequence of the associated event of unexpected seismic events (Sminchak and Gupta, 2002).
Possible health and safety risks related to CO2 streamA flow of substances resulting from CO2 capture processes, or which consists of a sufficient fraction of CO2 and sufficiently low concentrations of other substances to meet specifications of streams permitted for long term geological storage pressure and temperature include inhalation of, or exposure to, very cold air mixture, contact with solid CO2Carbon dioxide or cooled surfaces, rapid expansions, explosions and projectiles.