The leakage(in CO2 storage) The escape of injected fluid from the storage formation to the atmosphere or water column of CO2Carbon dioxide or the displacement of brine into a groundwater aquiferAn underground layer of fluid-bearing permeable rock or unconsolidated materials (gravel, sand, or silt) with significant permeability to allow flow produces significant changes in groundwater chemistry and quality and may also cause physical impacts by interfering with groundwater extraction procedures when a large plume of gaseous CO2Carbon dioxide is involved (Esposito and Benson, 2011). The contamination of groundwater with CO2Carbon dioxide would lead to the formationA body of rock of considerable extent with distinctive characteristics that allow geologists to map, describe, and name it of carbonic acid, which causes a decrease of pH, enabling dissolution of carbonateNatural minerals (e.g. calcite, dolomite, siderite, limestone) composed of various anions bonded to a CO32- cation minerals and mobilisation of trace chemicals.
For the Zero Emission Research and Technology (ZERT) experiment in Montana, USA, food grade CO2Carbon dioxide was injected over a period of one month during which an intensive monitoringMeasurement and surveillance activities necessary for ensuring safe and reliable operation of a CGS project (storage integrity), and for estimating emission reductions program, including water sampling, was performed. This experiment revealed significant changes in chemical parameters measured at the site, including a relatively rapid decrease of pH (7.0 to 5.6), increase of alkalinity (400 to 1,330 mg/l as bicarbonate, HCO3-), and electrical conductance (600 to 1,800 lS/cm) and an increase of natural trace chemicals (lead, arsenic, benzene) content following CO2Carbon dioxide injectionThe process of using pressure to force fluids down wells (Kharaka et al., 2010). The increased levels of contaminants in the groundwater could pose a serious threat to human health if the groundwater aquiferAn underground layer of fluid-bearing permeable rock or unconsolidated materials (gravel, sand, or silt) with significant permeability to allow flow is used as water supply or the reservoirA subsurface body of rock with sufficient porosity and permeability to store and transmit fluids is connected with water supplies.
Modelling CO2Carbon dioxide intrusion into groundwater aquifers revealed mobilisation of lead and arsenic as a result of the contamination, while for aquifers with low velocity, the impact of CO2Carbon dioxide intrusion can be more significant but is also more localised (Zheng et al., 2009).
Another aspect of CO2Carbon dioxide leakage(in CO2 storage) The escape of injected fluid from the storage formation to the atmosphere or water column into the groundwater is the interactions of coCarbon monoxide-injected/coCarbon monoxide-transported gases with the mineral matrix and the subsurface water. According to the modelling work of Harvey et al. (2012), there is a significant difference between the geochemical impact of a mixed gas (99.4% CO2Carbon dioxide, 0.1% COCarbon monoxide, 0.1% NOx, 0.3% SOx and 0.1% CH4) and a CO2Carbon dioxide-only stream (which cannot be obtained with the current captureThe separation of carbon dioxide from other gases before it is emitted to the atmosphere technologies) as the coCarbon monoxide-injected/coCarbon monoxide-transported gases could potentially influence aqueous speciationThe determination of the number of species into which a single species may evolve over time and mobility of redoxReduction-oxidisation reaction-sensitive elements such as Iron and Arsenic. CoCarbon monoxide-transported NOx or SOx into potable groundwater or soil pore water could contribute to additional lowering of the pH (up to 1 unit) that could lead to enhanced release of trace metals and formationA body of rock of considerable extent with distinctive characteristics that allow geologists to map, describe, and name it of carcinogenic nitroso compounds (from Nitrogen dioxide NO2 and organic matter) (Harvey et al., 2012). The same authors also proposed the conceptual framework for assessing geochemical impact of CO2Carbon dioxide on near surface environments, presented in the Fig. 3-2.
| 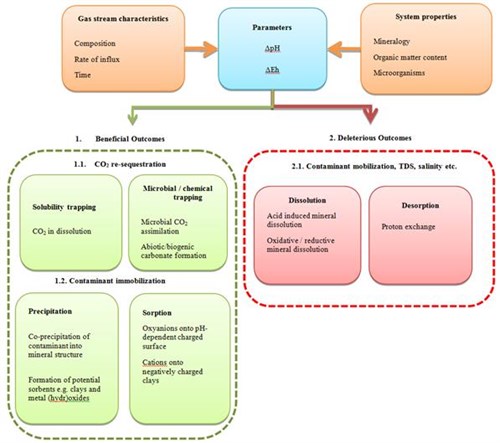
Fig. 3-2: A conceptual framework for assessing geochemical impact of CO2Carbon dioxide on near surface environments (after Harvey et al., 2012) |